Redox reactions are the last reactions we will study in this unit. It encompasses synthesis, decomposition, and single replacement reactions. It is categorized by all reactions that involve in a transfer of electrons.
 |
- If the element/compound loses electrons, it is oxidized (because the charge becomes more positive)
- If the element/compound gains electrons it is reduced (because the charge becomes more negative)
Always Remember OIL RIG!!!
Oxidation Is Lost
Reduction Is Gained
OIL RIG
The "lost" and "gained" refers to the electrons
- Also, to make things more confusing, the compound/element being oxidized is referred to as the reducing agent
- The compound/element being reduced is referred to as the oxidizing agent
- Just think about what that compound/element is doing to the other compound/element
- To determine the oxidation number of the element use these rules:
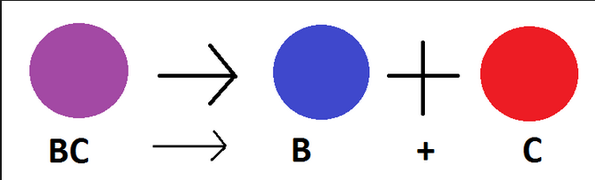
Single Replacement:
- Always follows this structure:
- The completion of the reaction is based upon the reactivity of the elements

If the element is less reactive than the element it is trying to "replace" in the reaction, the reaction will not be completed - Nonmetals replace nonmetals and metals replace metals
- For example:

The Fe (a metal) replaced the Cu (a metal) to create FeSO4
- Follows this structure:
- For example:
 |
| The H2 combines with the O2 to form H2O |
Decomposition:
Bozeman Science Redox Reactions Video

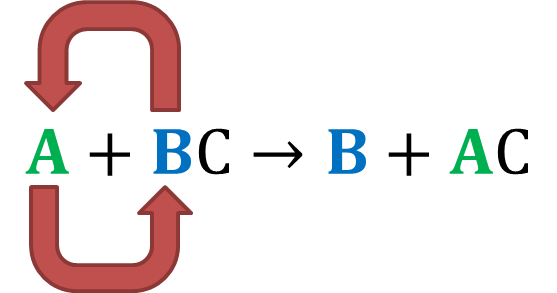
.png?revision=2)

Thanks, Laurie! This summarization over the redox lesson really helped. You went into detail for each type of reaction, but it wasn't too much where I felt overwhelmed like how I do when I read the textbook for review.
ReplyDeleteOh my goodness! Thanks so much for the breakdown of each and every type of redox! This lecture seemed so confusing up until the end, when she showed us that diagram of all of the reactions in relation to one and another. Hopefully this unit will help me improve on my connection and application skills, much like the last one.
ReplyDeleteThis summary/lesson of Redox was awesome! I wish I would have seen this before the test. It is really helpful to see the information to memorize how it all works, then connect it to application.
ReplyDeleteThis summary/lesson of Redox was awesome! I wish I would have seen this before the test. It is really helpful to see the information to memorize how it all works, then connect it to application.
ReplyDelete